The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units. The formula mass of a covalent compound is also called the molecular mass. A convenient amount unit for expressing very large numbers of atoms or molecules is the mole.
Experimental measurements have determined the number of entities composing 1 mole of substance to be 6.022 × 1023, a quantity called Avogadro's number. The mass in grams of 1 mole of substance is its molar mass. Due to the use of the same reference substance in defining the atomic mass unit and the mole, the formula mass and molar mass (g/mol) for any substance are numerically equivalent . The mole is a unit used to measure the number of atoms, molecules, or formula units in a given mass of a substance. The mole is defined as the amount of substance that contains the number of carbon atoms in exactly 12 g of carbon-12 and consists of Avogadro's number (6.022 × 1023) of atoms of carbon-12.
The molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 1023 atoms, molecules, or formula units of that substance. Of a substance is defined as the mass in grams of 1 mol of that substance. One mole of isotopically pure carbon-12 has a mass of 12 g. That is, the molar mass of a substance is the mass of 6.022 × 1023 atoms, molecules, or formula units of that substance. In each case, the number of grams in 1 mol is the same as the number of atomic mass units that describe the atomic mass, the molecular mass, or the formula mass, respectively. The molar mass of any substance is numerically equivalent to its atomic or formula weight in amu.
Per the amu definition, a single 12C atom weighs 12 amu . A mole of 12C weighs 12 g (its molar mass is 12 g/mol). This relationship holds for all elements, since their atomic masses are measured relative to that of the amu-reference substance, 12C. Extending this principle, the molar mass of a compound in grams is likewise numerically equivalent to its formula mass in amu (Figure 3.6). Of a substance is the sum of the average masses of the atoms in one molecule of a substance.
It is calculated by adding together the atomic masses of the elements in the substance, each multiplied by its subscript in the molecular formula. Because the units of atomic mass are atomic mass units, the units of molecular mass are also atomic mass units. The procedure for calculating molecular masses is illustrated in Example 1. As you learned in Chapter 1 "Introduction to Chemistry", the mass number is the sum of the numbers of protons and neutrons present in the nucleus of an atom. The mass number is an integer that is approximately equal to the numerical value of the atomic mass.
Although the mass number is unitless, it is assigned units called atomic mass units . The average mass of a monatomic ion is the same as the average mass of an atom of the element because the mass of electrons is so small that it is insignificant in most calculations. Many argue that modern chemical science began when scientists started exploring the quantitative as well as the qualitative aspects of chemistry. For example, Dalton's atomic theory was an attempt to explain the results of measurements that allowed him to calculate the relative masses of elements combined in various compounds.
Understanding the relationship between the masses of atoms and the chemical formulas of compounds allows us to quantitatively describe the composition of substances. We can argue that modern chemical science began when scientists started exploring the quantitative as well as the qualitative aspects of chemistry. Is the sum of the atomic masses of all the elements in the empirical formula, each multiplied by its subscript .
It is directly analogous to the molecular mass of a covalent compound. A The empirical formula—Ca32—indicates that the simplest electrically neutral unit of calcium phosphate contains three Ca2+ ions and two PO43− ions. The formula mass of this molecular unit is calculated by adding together the atomic masses of three calcium atoms, two phosphorus atoms, and eight oxygen atoms. To calculate the molecular mass of a covalent compound and the formula mass of an ionic compound and to calculate the number of atoms, molecules, or formula units in a sample of a substance.
The relationships between formula mass, the mole, and Avogadro's number can be applied to compute various quantities that describe the composition of substances and compounds. For example, if we know the mass and chemical composition of a substance, we can determine the number of moles and calculate number of atoms or molecules in the sample. Likewise, if we know the number of moles of a substance, we can derive the number of atoms or molecules and calculate the substance's mass. In an earlier chapter, we described the development of the atomic mass unit, the concept of average atomic masses, and the use of chemical formulas to represent the elemental makeup of substances. These ideas can be extended to calculate the formula mass of a substance by summing the average atomic masses of all the atoms represented in the substance's formula.
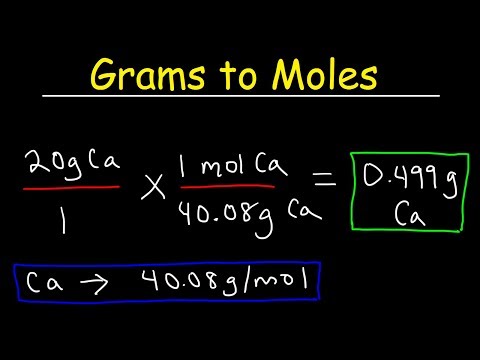
The mass of a mole of substance is called the molar mass of that substance. The molar mass is used to convert grams of a substance to moles and is used often in chemistry. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). If the mass of a substance is known, the number of moles in the substance can be calculated. Converting the mass, in grams, of a substance to moles requires a conversion factor of (one mole of substance/molar mass of substance). The identity of a substance is defined not only by the types of atoms or ions it contains, but by the quantity of each type of atom or ion.
For example, water, H2O, and hydrogen peroxide, H2O2, are alike in that their respective molecules are composed of hydrogen and oxygen atoms. However, because a hydrogen peroxide molecule contains two oxygen atoms, as opposed to the water molecule, which has only one, the two substances exhibit very different properties. This experimental approach required the introduction of a new unit for amount of substances, the mole, which remains indispensable in modern chemical science. Note that the average masses of neutral sodium and chlorine atoms were used in this computation, rather than the masses for sodium cations and chlorine anions. This approach is perfectly acceptable when computing the formula mass of an ionic compound.
Even though a sodium cation has a slightly smaller mass than a sodium atom , this difference will be offset by the fact that a chloride anion is slightly more massive than a chloride atom . Moreover, the mass of an electron is negligibly small with respect to the mass of a typical atom. The few exceptions to this guideline are very light ions derived from elements with precisely known atomic masses. The molar mass of any substance is its atomic mass, molecular mass, or formula mass in grams per mole. We want molecules of ethanol to answer the question. Now we need to come up with conversion factors to cancel units and get to the desired set of units.
How To Find Volume In Chemistry With Moles This can be used to cancel out the given number's units. In order to cancel units, multiply by the reciprocal of the molecular mass. Avogadro's number can be used to cancel out the moles and leaves molecules of ethanol units.
Also, remember to use the correct number of significant figures. The relative molecular mass of a compound is the sum of the relative atomic masses of all the atoms in a molecule of the compound. Each ion, or atom, has a particular mass; similarly, each mole of a given pure substance also has a definite mass. The mass of one mole of atoms of a pure element in grams is equivalent to the atomic mass of that element in atomic mass units or in grams per mole (g/mol).
Although mass can be expressed as both amu and g/mol, g/mol is the most useful system of units for laboratory chemistry. The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula. Moles are units used to measure substance amount. The use of these conversions is illustrated in Example 3 and Example 4.
Again, we used the same gram to mole conversion factor, the molecular mass, and also Avogadro's number to go from moles to atoms. Notice that there is an extra conversion in this equation. In each molecule of ethanol there are two carbon atoms. This also means that for each mole of ethanol there are two moles of carbon. In chemistry, a compound is a molecule consisting of at least two different types of atoms. All compounds are molecules, but not all molecules are compounds!
Ionic compounds, such as NaCl and KBr, do not form traditional discrete molecules like those formed by covalent bonds. In their solid state, these substances form a three-dimensional array of charged particles. In such a case, molecular weight has no meaning, so the term formula weight is used instead. Consistent with its definition as an amount unit, 1 mole of any element contains the same number of atoms as 1 mole of any other element.
The masses of 1 mole of different elements, however, are different, since the masses of the individual atoms are drastically different. The molar mass of an element is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (g/mol) . The mole is an amount unit similar to familiar units like pair, dozen, gross, etc.
It provides a specific measure of the number of atoms or molecules in a bulk sample of matter. A mole is defined as the amount of substance containing the same number of discrete entities as the number of atoms in a sample of pure 12C weighing exactly 12 g. One Latin connotation for the word "mole" is "large mass" or "bulk," which is consistent with its use as the name for this unit. The mole provides a link between an easily measured macroscopic property, bulk mass, and an extremely important fundamental property, number of atoms, molecules, and so forth. While atomic mass and molar mass are numerically equivalent, keep in mind that they are vastly different in terms of scale, as represented by the vast difference in the magnitudes of their respective units .
To appreciate the enormity of the mole, consider a small drop of water weighing about 0.03 g (see Figure 3.7). Although this represents just a tiny fraction of 1 mole of water (~18 g), it contains more water molecules than can be clearly imagined. If the molecules were distributed equally among the roughly seven billion people on earth, each person would receive more than 100 billion molecules. Mole is defined as the mass of the substance which consists of the equal quantity of basic units.
The basic units can be molecules, atoms or formula units based on the substance. The quantity of a substance that contains the same number of units (e.g., atoms or molecules) as the number of carbon atoms in exactly 12 g of isotopically pure carbon-12. Chemists can measure a quantity of matter using mass, but in chemical reactions it is often important to consider the number of atoms of each element present in each sample. Even the smallest quantity of a substance will contain billions of atoms, so chemists generally use the mole as the unit for the amount of substance.
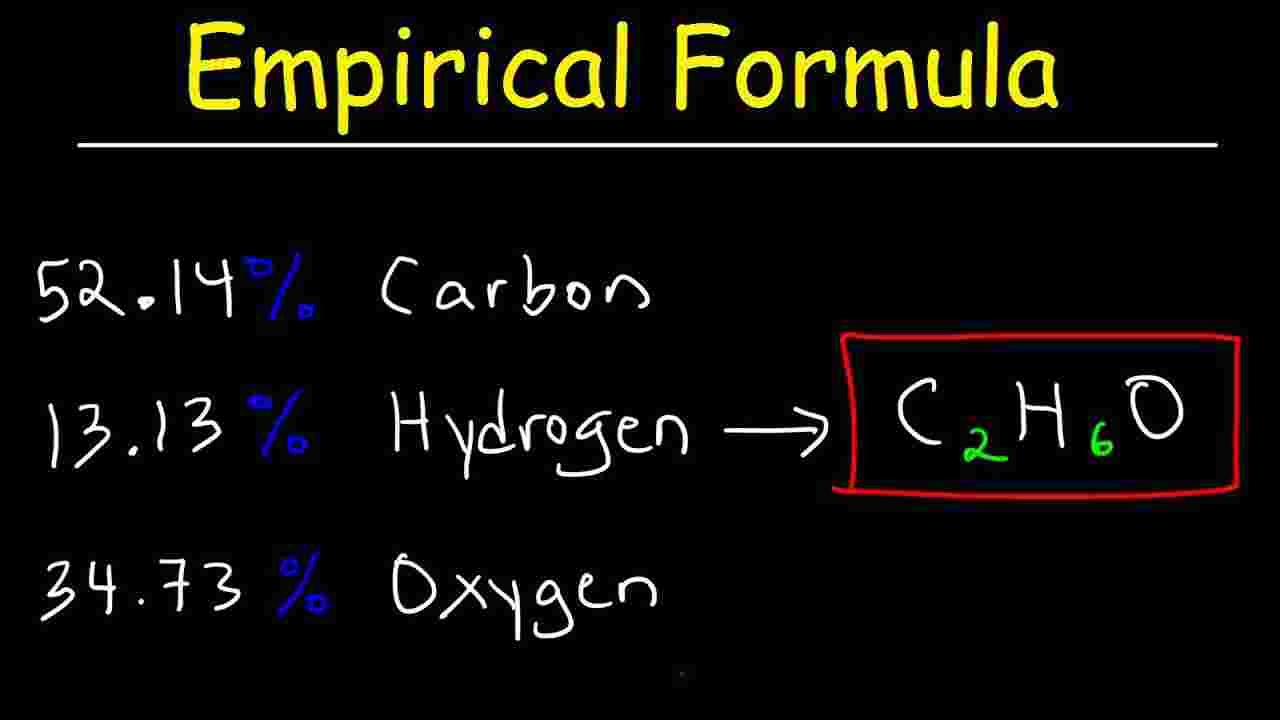
Avogadro's number is a proportion that relates molar mass on an atomic scale to physical mass on a human scale. Avogadro's number is defined as the number of elementary particles (molecules, atoms, compounds, etc.) per mole of a substance. It is equal to 6.022×1023 mol-1 and is expressed as the symbol NA. The empirical formula of a compound is the simplest form of the ration of the atoms of different elements in it. The molecular formula tells the actual number of each kind of atom in one molecule of the substance. The molecular weight of a molecule is calculated by adding the atomic weights of the atoms in the molecule.
The formula weight of an ionic compound is calculated by adding its atomic weights according to its empirical formula. The characteristic molar mass of an element is simply the atomic mass in g/mol. However, molar mass can also be calculated by multiplying the atomic mass in amu by the molar mass constant (1 g/mol). To calculate the molar mass of a compound with multiple atoms, sum all the atomic mass of the constituent atoms. The mole is the SI measure of quantity of a "chemical entity," such as atoms, electrons, or protons. It is defined as the amount of a substance that contains as many particles as there are atoms in 12 grams of pure carbon-12.
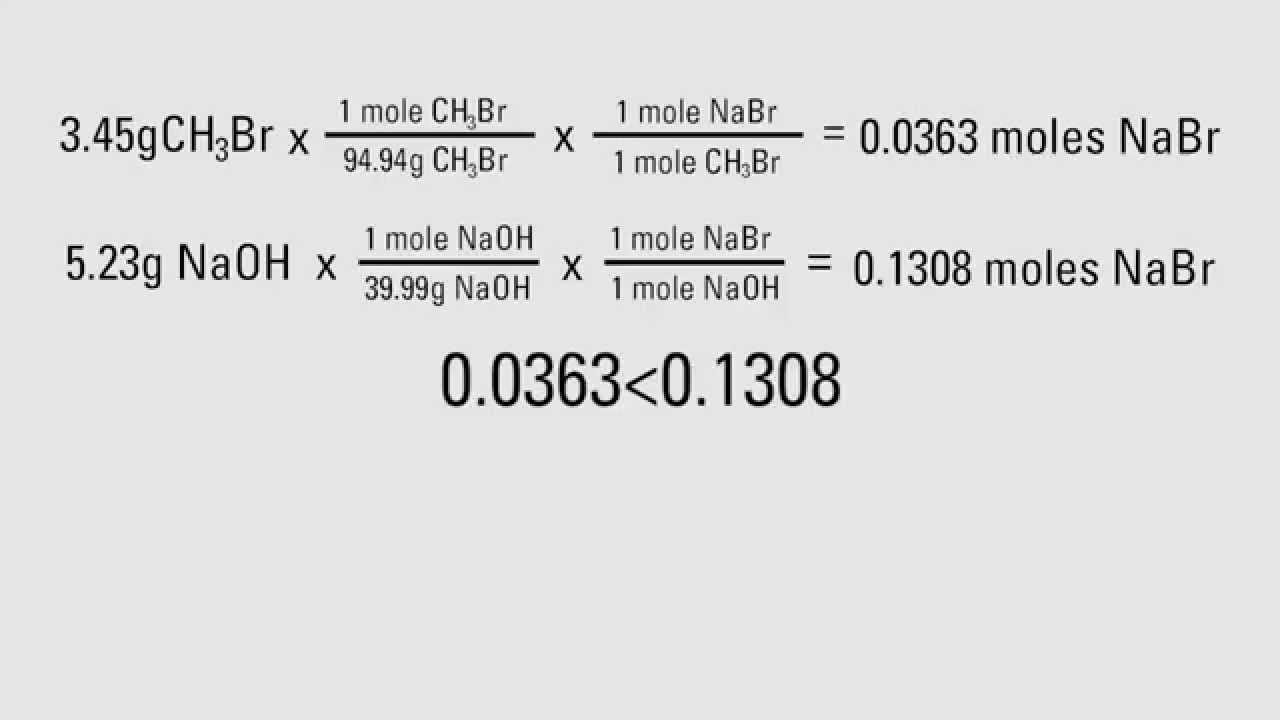
So, 1 mol contains 6.022×1023 elementary entities of the substance. Note that since relative atomic masses are ratios they have no units. Use the molecular formula to find the molar mass; to obtain the number of moles, divide the mass of compound by the molar mass of the compound expressed in grams.
We can also use chemical formulas as conversion factors to convert from moles of a compound to moles of an element in the compound . Therefore, a mole is defined as the amount of substance containing the same number of discrete entities as the number of atoms in a sample of pure 12C weighing exactly 12 g. A Use the molecular formula of the compound to calculate its molecular mass in grams per mole. The molar mass of a compound is equal to the sum of the atomic masses of its constituent atoms in g/mol. The molar mass of a compound can be calculated by adding the standard atomic masses (in g/mol) of the constituent atoms. The chemical changes observed in any reaction involve the rearrangement of billions of atoms.
It is impractical to try to count or visualize all these atoms, but scientists need some way to refer to the entire quantity. They also need a way to compare these numbers and relate them to the weights of the substances, which they can measure and observe. The solution is the concept of the mole, which is very important in quantitative chemistry.
A typical human brain weighs about 1.5 kg and occupies a volume of roughly 1.1 L. Chemical signaling occurs at the interface between different neurons when one of the cells releases molecules that diffuse across the small gap between the cells and bind to the surface of the other cell. These neurotransmitter molecules are stored in small intracellular structures called vesicles that fuse to the cell wall and then break open to release their contents when the neuron is appropriately stimulated. One neurotransmitter that has been very extensively studied is dopamine, C8H11NO2. Dopamine is involved in various neurological processes that impact a wide variety of human behaviors. Dysfunctions in the dopamine systems of the brain underlie serious neurological diseases such as Parkinson's and schizophrenia.
Values for atomic and therefore molecular masses are normally obtained relative to the mass of the isotope 12C (carbon-12), however "relative" is generally omitted from the title. Written correctly, the relative values have no units. Many familiar items are sold in numerical quantities that have unusual names. For example, cans of soda come in a six-pack, eggs are sold by the dozen , and pencils often come in a gross . Sheets of printer paper are packaged in reams of 500, a seemingly large number.
Atoms are so small, however, that even 500 atoms are too small to see or measure by most common techniques. Any readily measurable mass of an element or compound contains an extraordinarily large number of atoms, molecules, or ions, so an extraordinarily large numerical unit is needed to count them. Of a substance by summing the average atomic masses of all the atoms represented in the substance's formula. Similarly, if the moles of a substance are known, the number grams in the substance can be determined. Converting moles of a substance to grams requires a conversion factor of molar mass of substance/one mole of substance. One simply needs to follow the same method but in the opposite direction.
Be particularly careful when looking at the mass of an element in a compound. Remember, the only way you can change from molecule to atoms, or vice-versa, is by using the chemical formula, and the chemical formula relates ONLY to particles or moles, not to mass. We can calculate the total molar mass of a compound the same way we calculated total molecular mass—just replace amu with grams —but keep in mind the difference between what these two terms mean. Mass of one atom of that element which typically reflects the mass of the nucleus .


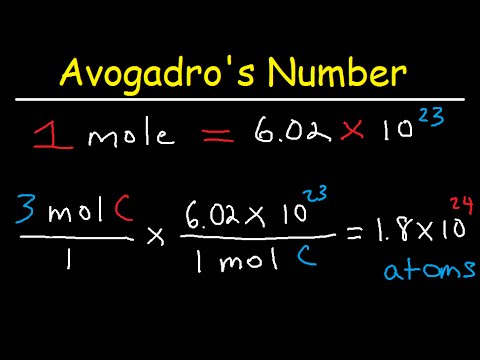















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.